Lipoprotein trafficking, the key to building an outer membrane barrier
Several molecular machines have been discovered that stitch together individual lipid and protein molecules into a robust outer membrane barrier. The Bam machine folds and inserts transmembrane proteins (termed OMPs) in the outer membrane; OMPs allow nutrients to enter the cell and also enable efflux of toxins and antibiotics out of the cell. The Lpt machine transports lipopolysaccharide (LPS) to the external surface of the outer membrane. This layer of LPS molecules fortifies the outer membrane through intermolecular interactions that seal the lipid bilayer.Lipoproteins (a family of lipid-anchored proteins) are essential components in each of the outer membrane assembly machines. However, lipoproteins are made in the inner membrane and must be trafficked to the outer membrane. As a result, Gram-negative bacteria rely on efficient trafficking of all these lipoproteins to supply the outer membrane assembly machines. Lipoproteins also play fundamental roles in building the peptidoglycan cell wall, antibiotic efflux, secretion of proteins and polysaccharides, as well as contributing in diverse ways to virulence of pathogens.
The LolAB route has been the only known trafficking pathway by which lipoproteins are brought to the outer membrane. We discovered that another route exists. Our insights guide the pursuit of new antibiotics that will disrupt lipoprotein trafficking, weakening the outer membrane barrier and killing bacteria.
We are working to develop a more complete understanding of how essential lipoproteins reach the outer membrane
Envelope stress responses that monitor outer membrane assembly
Outer membrane assembly is a complex process that is continuously monitored by stress-responsive signal transduction systems. The Cpx system helps pathogens to adapt when challenged by antibiotics. NlpE (pictured) is a sensory outer membrane lipoprotein that helps to activate the Cpx stress response.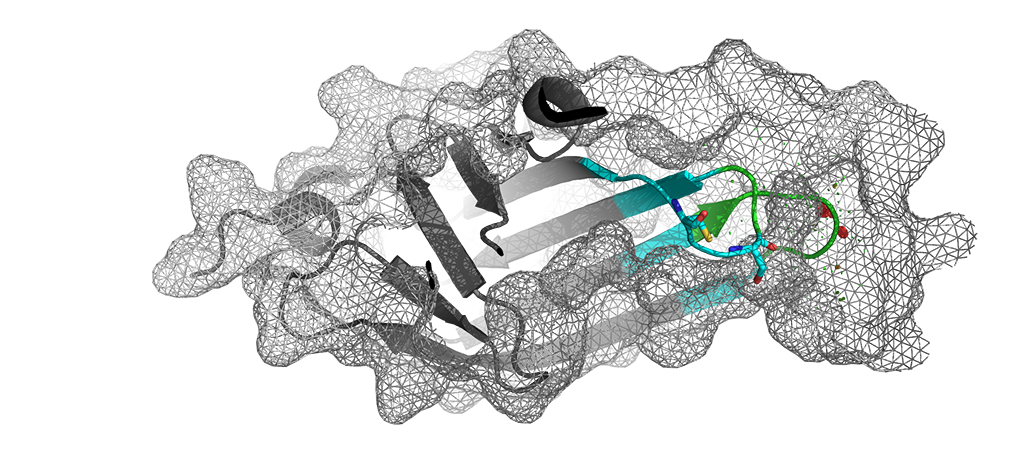
We are studying the Cpx response to discover how it protects bacteria against stress
Vaccine adjuvant development
We discovered an unusual molecule that covalently couples two potent activators of innate immunity, lipopolysaccharide (LPS) and peptidoglycan (PG). A modified E. coli strain makes such molecules as part of an unusual strategy for resistance against the antibiotic vancomycin. Vancomycin targets PG precursors that are normally shielded beneath the outer membrane. In mutants that produce a leaky outer membrane, vancomycin can enter the cell and reach the target PG precursors. A single mutation (waaL15) allows E. coli to make LPS-PG hybrid molecules that are transported to the cell's surface. LPS-PG molecules bringing the vancomycin target outside of the cell and act as decoys for vancomycin, sequestering the antibiotic harmlessly outside the cell (green surface binding below).Both LPS and PG are potent activators of immunity. The presence of both molecules synergistically improves immune activation.
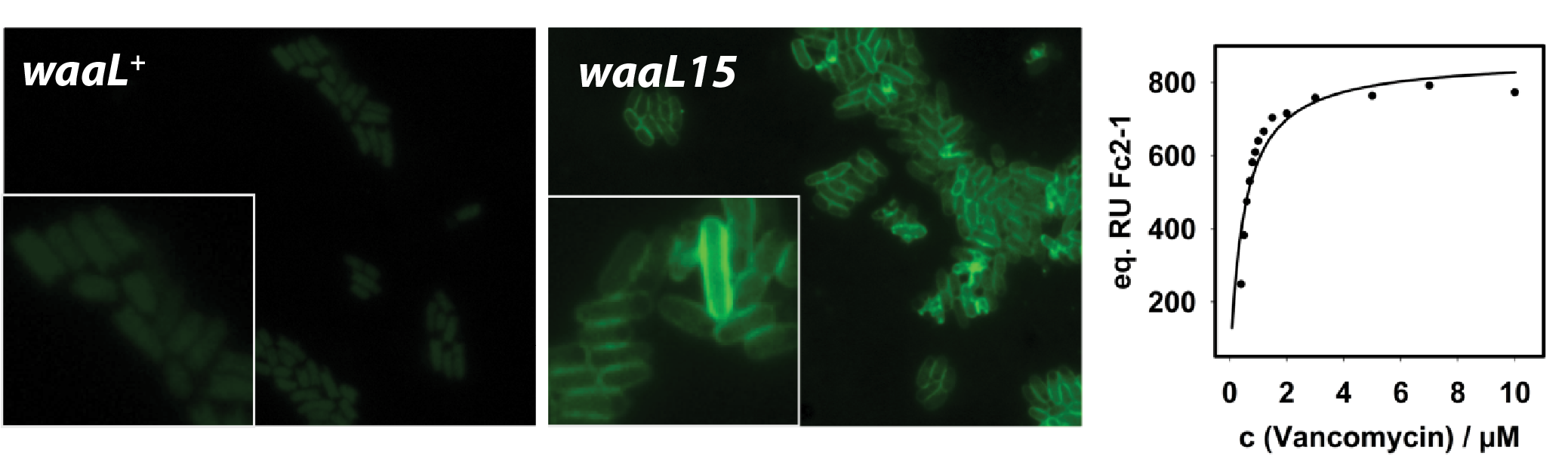
We are investigating the properties of LPS-PG hybrid molecules with the aim of developing new vaccine adjuvants